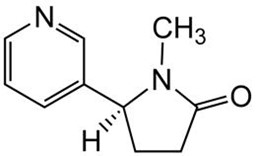
(-)-Cotinine.HCl
(-)-Pseudoephedrine
(+)-Ephedrine.HCl
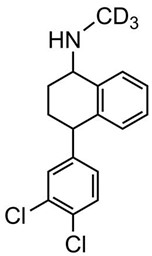
(±)-cis-Sertraline-D3.HCl
1,25-Dihydroxyvitamin D3
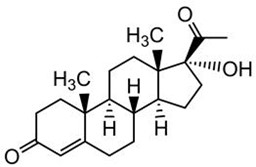
17alpha-Hydroxyprogesterone
25C-NB2OMe.HCl
Latest Entries
Click here to find the latest product news at a glance:
News Flyer - September 2021
LIPOMED is currently transitioning to new labels featuring an innovative transfer part.
Our transfer label can be easily detached from the original LIPOMED ampoule to an auto sampler vial providing an easy, safe and accurate identification of the vial once the original ampoule has been opened. LIPOMED transfer labels will be provided for all our calibrated reference standards in solution.
Please see our flyer for more information.
Aqueous Ethanol Standards
LIPOMED announces the availability of its new product line of AQUEOUS ETHANOL STANDARDS. LIPOMED has developed this line to support laboratories looking for high quality and reliable aqueous ethanol standards used in the calibration of analytical techniques for quantitative analysis of ethanol.
Commitment to Quality
LIPOMED AG, Switzerland is GMP, GDP and ISO 9001 certified for production and distribution of Pharmaceuticals and ISO 17025 accredited for testing of Analytical Reference Standards and ISO 17034 accredited for Reference Material production. All products distributed by LIPOMED affiliates are manufactured by LIPOMED AG and distributed under protocols of LIPOMED AG.
Stay up to date
If you are interested in receiving our newsletter to receive updates on new products, please send us an e-mail to Lipomed.EMEA@lgcgroup.com.
The newsletter is published on a regular basis in english, french and german.